Kayayyaki
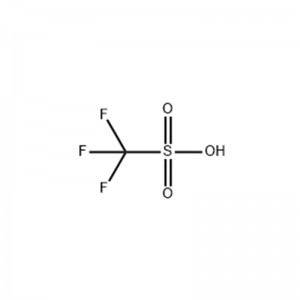
Trifluoromethanesulfonic acid
Tsarin Tsari
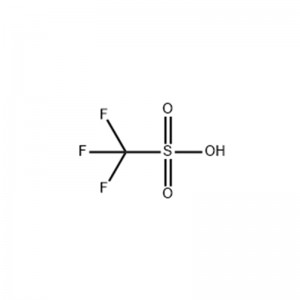
Abubuwan Jiki
Bayyanar: Ruwa mai launin ruwan rawaya
Yawa: 1.696 g/mL a 25 ° C (lit.)
Matsayin narkewa: -40 ° C
Matsayin tafasa: 162 ° C (lit.)
Refractivity: n20/D 1.327(lit.)
Ma'anar walƙiya: Babu
Adadin acidity (pKa): -14 (at25 ° C)
Musamman nauyi: 1.696
Ƙimar PH:<1 (H2O)
Bayanan Tsaro
Nasa ne na kayan haɗari
Rukunin haɗari: 8
Lambar jigilar kayayyaki mai haɗari: UN 3265 8/PG 2
Rukunin tattarawa: II
Lambar Kwastam: 2904990090
Ƙimar Maido Harajin Fitarwa (%): 9%
Aikace-aikace
Shi ne mafi ƙarfi sananne Organic acid da kuma m roba kayan aiki.Tare da karfi corrosivity da hygroscopicity, shi ne yadu amfani a Pharmaceutical da kuma sinadaran masana'antu, kamar nucleosides, maganin rigakafi, steroids, gina jiki, saccharides, bitamin kira, silicone roba gyara, da dai sauransu Tare da kananan sashi, karfi acidity da barga Properties, shi zai iya maye gurbinsu. inorganic acid na gargajiya irin su sulfuric acid da hydrochloric acid a lokuta da yawa kuma suna taka rawa wajen ingantawa da inganta tsarin.Hakanan za'a iya amfani dashi azaman mai haɓakawa don isomerization da alkylation don shirya 2,3-dihydro-2-indenone da 1-tetralone, da kuma cire glycoside daga glycoprotein.
Kariyar Tsaro
Trifluoromethanesulfonic acid yana daya daga cikin mafi karfi kwayoyin acid.Haɗuwa da idanu zai haifar da ƙonewa mai tsanani da kuma yiwuwar makanta.Tuntuɓar fata zai haifar da ƙona sinadarai mai tsanani, da kuma jinkirta mummunan lalacewar nama.Shakawar tururi na iya haifar da mugunyar jijiyoyi, kumburi, da edema.Ciwon ciki na iya haifar da kunar ciki mai tsanani.Saboda haka, ko da ƙananan kuɗi suna buƙatar kayan kariya masu dacewa (kamar tabarau, safofin hannu na acid da alkali, da abin rufe fuska na gas), da samun iska mai kyau.
Bugu da kari na trifluoromethanesulfonic acid zuwa iyakacin duniya kaushi yana haifar da exotherm saboda rushewa.Wannan matsanancin exotherm yayi kama da tasirin sulfuric acid a cikin ruwa.Duk da haka, narkar da shi a cikin ruwan lemo yana da haɗari a zahiri fiye da narkar da sulfuric acid a cikin ruwa.Ƙarfin ƙaƙƙarfan ƙaƙƙarfan ƙaƙƙarfan ƙaƙƙarfan ƙaƙƙarfan ƙaƙƙarfan ƙaƙƙarfan ƙaƙƙarfan ƙaƙƙarfan ƙaƙƙarfan ƙaƙƙarfan ƙaƙƙarfan ƙaƙƙarfan ƙaƙƙarfan ƙaƙƙarfan ƙaƙƙarfan ƙaƙƙarfan ƙaƙƙarfan ƙaƙƙarfar ƙaƙƙarfan ƙaƙƙarfan ƙaƙƙarfan ƙaƙƙarfan ƙaƙƙarfan ƙaƙƙarfan ƙaƙƙarfan ƙaƙƙarfan ƙaƙƙarfan ƙaƙƙarfan ƙaƙƙarfan ƙaƙƙarfan ƙaƙƙarfan ƙaƙƙarfan ƙaƙƙarfan ƙaƙƙarfan ƙaƙƙarfan ƙaƙƙarfan ƙaƙƙarfan ƙaƙƙarfan ƙaƙƙarfan ƙaƙƙarfan ƙaƙƙarfan ƙaƙƙarfan ƙaƙƙarfan ƙaƙƙarfan ƙaƙƙarfan ƙaƙƙarfan ƙaƙƙarfan ƙaƙƙarfan ƙaƙƙarfan ƙaƙƙarfan ƙaƙƙarfan ƙaƙƙarfan ƙaƙƙarfan ƙaƙƙarfan ƙaƙƙarfan ƙaƙƙarfan ƙaƙƙarfan ƙaƙƙarfan ƙaƙƙarfan ƙaƙƙarfan ƙaƙƙarfan na iya haifar da ƙanƙara don ƙafe ko ma fashe.Saboda haka, ya kamata a kauce wa narkar da adadi mai yawa na trifluoromethanesulfonic acid a cikin kaushi na kwayoyin halitta.Lokacin da ya zama dole don yin haka, tabbatar da sarrafa saurin digo kuma tabbatar da isassun motsawa, samun iska mai kyau, da yuwuwar sanyaya na'urorin musayar don cire yawan zafin da aka haifar gwargwadon yiwuwa.